Personalised vs Generic Cardiac Resuscitation
The resuscitation guidelines have changed little from their introduction in the 1960’s. We’ve changed the ratio of compressions to breaths, we’ve used adrenaline, had a short fling with vasopressin and returned to adrenaline again. Now the effectiveness of adrenaline outside the first 9 or 10 minutes of a resuscitation is being questioned. Perhaps Advanced Cardiac Life Support (ACLS) gives us little more than Basic Life Support (BLS).
The problem with the guidelines is that they are generic, a one size fits all approach. We know that patients are not all the same, their comorbidities are not all the same and their reasons for having a cardiac arrest are not all the same. We also know that they will not all respond in the same way.
We have seen the introduction of Extracorporeal Membrane Oxygenation (ECMO), with some incredible results, but have realised that it is currently a limited resource and that we must concentrate on improving what we have, certainly for now. To do this, we have concentrated on improving what we do with airway, by not intubating early, especially if it interferes with Cardiopulmonary Resuscitation (CPR). We’ve become far more aware of how well we need perform CPR, because is one of the most important things we can get right in a resuscitation, that will potentially have the greatest effect on outcome. We are still missing the most important thing; tailoring what we do for that particular patient.
It’s now time to move away from a rescuer-centric model where we concentrate on breaths and duty cycles and depth of compression and to a patient-centric model, where the focus is on tailoring our resuscitation to address the physiology of that patient, in real time. We need to move to measuring Diastolic Blood Pressures (DBP), Coronary perfusion Pressures (CoPP), End Tidal Carbon Dioxide (EtCO2) and using real time Echocardiography (ECHO) to guide our resuscitation.
There are two theories relating to what happens when we perform CPR:
- The Cardiac Pump Theory: Chest compressions directly compress the ventricles generating blood flow.
- The Thoracic Pump Theory: The increase and decrease in intrathoracic pressure induces venous return to the heart.
We aren’t quite sure how much each contributes, however it’s certainly a combination of both. With our best efforts CPR we will attain, at most, 50% of normal cardiac output, usually termed, the low flow state. It’s the best means we have of generating perfusion, we have for now.
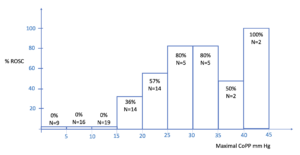
Current recommendations are that we should aim for a DBP of greater than 25mmHg and a CoPP of greater than 20mmHg. Coronary perfusion occurs during the relaxation phase of compressions and is due to blood passively flowing from the aorta into the coronary arteries. Maximal CoPP is the best predictor of Return of Spontaneous Circulation (ROSC) and there appears to be a proportional relationship (1)
What are the Anatomical Landmarks for Chest Compressions?
In tailoring our approach to each patient we need to move away from the generic approach to CPR. We can concentrate on depth and duty cycle in cardiac compressions, but are we being effective? The current recommendation is that we compress the lower half of the sternum, using the level of the inter-nipple line as a landmark (2). This is a rescuer-centric approach that we need to improve, because it assumes that the heart is in the same position for everyone. Surely the position of the heart in a 140kg obese patient and a 60kg patient, or in someone with COPD, cannot be in the same position.
What is under the inter-nipple line and is this where we should be compressing?
Shin (3) found that the left ventricle was at this level in only 20% of patients. In 80% of cases, using these landmarks, resulted in compression of the cardiac base, which included:
- The Left Ventricular Outflow Tract
- The Root of the Aorta
- The Ascending Aorta
 Transoesophageal ECHO used on intubated patients during cardiac arrest resuscitation (4), found that in 79% of cases the area of maximal compression was within 2cm of the aortic valve. Compression of the left ventricular outflow tract resulted in narrowing of the aortic root and in some cases obliterated the aorta during compression.
Transoesophageal ECHO used on intubated patients during cardiac arrest resuscitation (4), found that in 79% of cases the area of maximal compression was within 2cm of the aortic valve. Compression of the left ventricular outflow tract resulted in narrowing of the aortic root and in some cases obliterated the aorta during compression.
It appears that the inter-nipple line may not be the best landmark to use to perform cardiac compressions. It is considered a safe option, as performing lower or lateral chest compressions may injure the liver and ribs respectively. However, ineffective compression may be the greater evil.
The use of ECHO to guide compressions in real time during a resuscitation is not a new concept but one not widely used. We use ECHO during the compression breaks to look for cardiac motion, but not during the compressions themselves. Two liming factors may be that transoesophageal ECHO is not available to everyone and transthoracic ECHO interferes with the access to the chest wall.
Can we use a subcostal ECHO approach? Skulec (5) performed a prospective observational study in out of hospital cardiac arrest patients and used subcostal 4 chamber views during cardiac compressions to identify areas of maximal compression. In this study they were able to get adequate views in half of their patients, which allowed an evaluation of the degree of compression of the right and left ventricles.
ECHO used in this way, can determine the best position and depth to achieve maximal and correctly targeted cardiac compressions. Current guidelines recommend a fixed depth of 5cm, however we know that blood pressure varies amongst individuals with this depth. Some patients require a greater compression depth and some less.
There is a definite association between chest compression depth and systolic and diastolic blood pressures (6). However there is significant variation in individuals and inconsistent changes in invasive blood pressures measured, regardless of the compression depth. Steill (7) demonstrated that a depth less than 50mm (40.5-55.3mm) was better for survival to hospital discharge. Other studies (8) have shown survival with good functional outcome to be better when compressions are at least 50mm. We also know that at compressions depths greater than 60mm are associated with injuries (9).
There is no doubt that the variation in blood pressure response is multifactorial and may include the aetiology of the arrest, the patient’s pre-morbid condition, the time to CPR and the total amount of time CPR has been administered. However, there is also a real possibility that it is related to our point of maximal compression and where this is in relation to the cardiac chamber’s position in the chest. Real-time ECHO, using a subcostal approach may not be possible for everyone, as factors such as body habitus may affect the quality of images possible. However, it is a simple, non-invasive approach that can assist us in improving blood pressures, which have a direct effect on ROSC (1).
Something to consider during your next resuscitation
REFERENCES
- Paradis NA etal. Coronary Perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 1990;263:1106-1113.
- Callaway C et al. Advanced Life Support. 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment recommendations. Circulation 132(16) 2015.
- Shin J et al. Is the inter-nipple line the correct hand position for effective chest compression in adult cardiopulmonary resuscitation? Resuscitation. 2007;75:305-310
- Hwang S O et al. Compression of the Left Ventricular Outflow Tract during Cardiopulmonary Resuscitation. Acad Emerg Med. 2009. 16;10:928-933.
- Skulec R et al. Correlation between end-tidal carbon dioxide and the degree of compression of heart cavities measured by transthoracic echocardiography during cardiopulmonary resuscitation for out of hospital cardiac arrest. Critical Care 2019. 23;334:1-10.
- Sainio M et al. Simultaneous beat to beat assessment of arterial blood pressure and quality of cardiopulmonary resuscitation in out of hospital and in hospital settings. Resuscitation 2015;96:163-169
- Stiell IG et al. What is the optimal chest compression depth during out of hospital cardiac arrest resuscitation of adult patients? Circulation 2014;130:1962-1970.
- Vadeboncoeur T et al. Chest compression depth and survival in out of hospital cardiac arrest. Resuscitation 2014;85:182-8
- Helluvuo H et al. Deeper chest compressions-more complications for cardiac arrest patients? Resuscitation 2013;84:760-5.