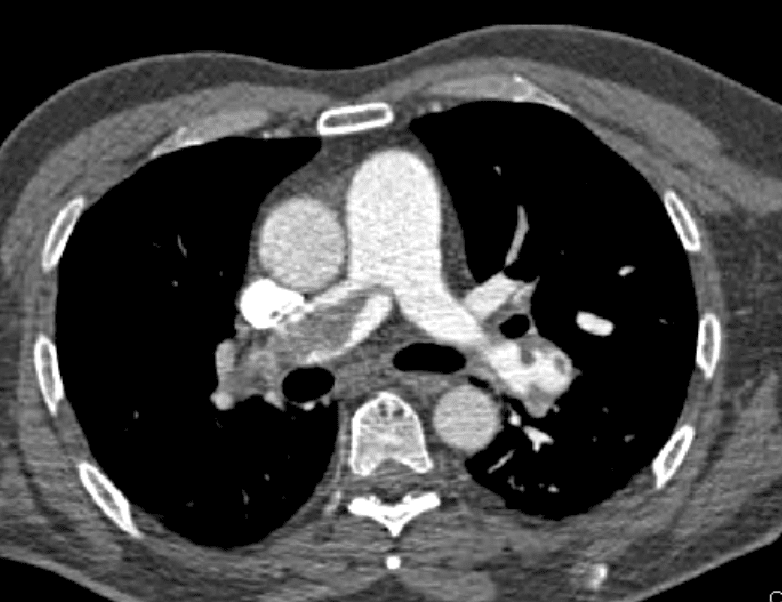
CASE
A 64 yo patient is referred to the emergency department(ED) with a persistent tachycardia. He was receiving chemotherapy as an outpatient, when nurses noted the abnormally fast heart rate. When assessed in the ED, he has a sinus tachycardia of 132 bpm, oxygen saturations of 85% on room air and he mentions a 2 week history of increasing shortness of breath. He is afebrile and has a blood pressure is 135/78 mm Hg. Clinical examination is essentially normal, with dual heart sounds and normal breath sounds, a soft non-tender abdomen and no leg swelling. His lab results show a normal full blood count and normal renal function. However the high sensitivity troponin is raised to 10 times the upper limit of normal.
Nasal prongs at 3L/min are applied and his heart rate decreases and saturations rise.
You working diagnosis is a pulmonary embolism(PE).
Below we review the approach to making the diagnosis and the treatment of patients with differing severity of PE(1) and aim to answer the following questions:
- How do we risk stratify patients with PE?
- How do we classify the severity of a PE and what is the treatment for each severity type?
- Do we treat sub segmental PE’s?
- Do we treat patients where the PE is asymptomatic and incidentally found?
- How long should treatment continue for?
- Is treatment different for patients with differing risk factors such as malignancy, pregnancy, anti-phospholipid syndrome or others?
- Which patients can be treated as outpatients?
Introduction
Patients with a PE have a mortality approaching 5% if undiagnosed…. and yes there are patients with PE who are asymptomatic. Most PE’s result from venous thromboembolism (VTE) of either the lower limbs(majority) or upper limbs.
Clinical Presentation and Diagnosis
Most patients presenting to the emergency department will have tachycardia, dyspnoea, chest pain, cough, haemoptysis, or syncope.
Our approach will depend on our level of suspicion (our gestalt) and thus our pre-test probability. In the case given above we have a high suspicion for a PE, based on the risk factors of malignancy and the clinical presentation of tachycardia, dyspnoea and hypoxia. In this patient, we progressed directly to a CT pulmonary angiogram (CTPA). A low risk gestalt, in contrast, is equivalent to a likelihood of a PE being lower than 15%. We can arrive at this by using rules and use the rules to determine if further investigation is needed.
Rules
The Pulmonary Embolism Rule-Out Criteria (PERC) rule allows us to rule out a PE when our gestalt says that there is a less than 15% chance of a PE being present. All parts of the PERC rule must be negative to rule out a PE. The clinician must be able to answer ‘NO’ to all of the following questions:
- Is the patient older than 49 years of age?
- Is the pulse rate above 99 beats min at any time?
- Is the pulse oximetry reading <95% on room air?
- Is there a present history of hemoptysis?
- Is the patient taking exogenous oestrogen?
- Does the patient have a prior diagnosis of VTE?
- Has there been recent surgery or trauma? (Endotracheal intubation / hospitalisation in the previous 4 weeks.)
- Does the patient have unilateral leg swelling?
When used in this way, the “combination of gestalt estimate of low suspicion for PE and PERC(-) reduces the probability of VTE to below 2% in about 20% of outpatients with suspected PE.” (3)
If the patient is unsuitable for the PERC rule, other rules can guide decision making. These rules generally classify the presence of PE as likely or unlikely and are used with D-Dimer testing, to determine the need for further investigations.
The Wells Score
The Wells score helps us risk stratify patients and can reduce the number of CTPAs performed. It is used in association with a D-dimer, to determine the need for further investigations. Like the D-dimer, it should not be used on all patients presenting with chest pain or dyspnoea, or leg swelling.
The Wells Score can help us risk stratify patients into low, intermediate, or high probability. When used with a D-dimer to rule out a PE, we use it to classify patients into one of two groups: likely or unlikely to have a PE.
The Wells score comprises the following:
- Clinical Signs and Symptoms of a DVT (3 points)
- PE as the #1 diagnosis OR Equally likely ( 3 points)
- Heart rate > 100 (1.5 points)
- Immobilisation for at least 3 days OR surgery in previous 4 weeks (1.5 points)
- Previously diagnosed PE or DVT (1.5 points)
- Haemoptysis (1 point)
- Malignancy with treatment in last 6 months or palliative (1 point)
A quantitative D-dimer can be used to rule out a PE in those with a Wells score of <4
The patient presented above, has a Wells score of 5.5 points. He is not low risk and D-dimer testing cannot be used to rule out PE. Definitive imaging is needed. If a D-dimer was done prior to risk stratification, it would most likely be raised anyway, due the patient’s malignancy.
The Revised Geneva Score
The Revised Geneva Score also allows risk stratification, which can then be used with a D-dimerto rule out a PE.
- Age > 65 (1 point)
- Previous PE/DVT (3 points)
- Surgery or lower limb fracture of the last month (2 points)
- Malignant condition; active or cured < 1 year (2 points)
- Unilateral lower limb pain (3 points)
- Haemoptysis (2 points)
- Heart Rate
- <75 (0 points)
- 75-94 (3 points)
- >94 (5 points)
- Pain on palpation of lower limb and unilateral oedema (4 points)
Low risk groups are defined as < 4 points and these patients have a 7-9% incidence of PE. A negative D-dimer in the low risk group can rule out a PE.
Our above would score 7 points, which puts him in the moderate risk group with a 20-30% chance of PE.
D-Dimer
The D-dimer can be used in low risk patients where our clinical suspicion of disease is low, to rule out PE. A D-dimer must not be performed prior to patient assessment and formulation of a pre-test probability, as it may be raised due to a large number of other medical conditions.
Various proposals for D-dimer cut offs have been made (4-7). An age adjusted D-dimer approach, of age x 10 can be used (sensitivity of 97%-99%).
YEARS Algorithm
The YEARS algorithm (8) is a new approach to using the D-Dimer. It uses a clinical decision rule which consists of 3 criteria:
- Clinical Signs of Deep Venous Thrombosis
- Haemoptysis
- Is Pulmonary Embolism the Most Likely Diagnosis?
What they found was that:
- In any patient with NO CRITERIA a D-dimer of <1000 ruled out PE
- In any patient WITH CRITERIA a D-dimer of < 500 rule out PE
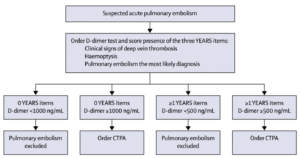
Imaging for Suspected Pulmonary Embolism.
There is a long standing discussion about CTPA vs V/Q scan in the investigation of PE. This is certainly an issue when related to the pregnant patient with a suspected PE.
CTPA has become the investigation of choice, due to its availability. Watch How to read a CTPA.
Risk Stratification of PE
A risk profile is assigned based on haemodynamic stability, right heart strain and elevated cardiac markers. Risk category directs treatment.
High Risk PE
High risk patients are haemodynamically unstable with systolic BP < 90mm Hg.
Intermediate Risk PE
These are patients with:
- Right heart strain shown by echocardiography or CT and
- Elevated cardiac markers such as troponin or brain natriuretic peptide.
Low Risk PE
This group of patients has no heart strain and normal cardiac markers.
Treatment
High Risk Patients
Treatment of the haemodynamically compromised patient is usually by intravenous systemic thrombolysis (9,10,11). This is preferred to catheter directed thrombolysis. Thrombolytics that can be used include Tenecteplase and Alteplase
Intermediate Risk Patients
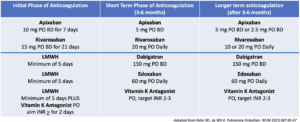
This group should receive anticoagulation. Low molecular weight heparin(LMWH) for approximately 5 days, is the initial agent of choice. Unfractionated Heparin can cause bleeding and we are unsure about direct oral anticoagulants (DOAC) versus LMWH for initial therapy. DOACs can be used following initial treatment.
Low Risk patients
These patients can initially be treated with a DOAC (9,10,11,12).
The choice of DOAC will depend on the clinical situation (see table below). In patients with advanced renal or liver disease or antiphospholipid syndrome, Vitamin K antagonists are preferred.
Dosages
Do we treat incidentally found, asymptomatic PE?
Both the American Society of Hematology(10) and the European Society of Cardiology (11) suggest anticoagulation in patients with malignancy. The American College of Chest Physicians (9) suggests similar treatment to that for symptomatic patients with a PE.
Do we treat sub-segmental PE?
This is a developing area. Anticoagulation is currently recommended for high risk patients, such as those with malignancy(9, 10) and for those patients who are hospitalised, immobile or with an unprovoked PE, or pregnant(9).
Which patients are suitable for discharge?
All high and intermediate risk patients need admission. Low risk patients can be assessed for their suitability for discharge, keeping in mind that in some cases this discharge may not be in the first 24 hours. The Hestia Criteria or the Simplified PESI Score can be used to assist decision making for discharge.
- The HESTIA Criteria: The patient must not be positive for any of the following criteria.
- Haemodynamically unstable (SBP <100mm Hg and HR > 100)
- Thrombolysis or embolectomy needed
- Active bleeding or high risk of bleeding
- GI Bleed or surgery < 2 weeks ago
- stroke < 1 month ago
- Bleeding disorder or platelet count < 75
- Uncontrolled hypertension > 180/>110
- > 24 hours of oxygen needed to maintain SaO2 > 90%
- Pe diagnosed whilst on anticoagulation
- Severe pain needing IV medication for > 24hours
- Medical or social reasons for admission
- Creatinine clearance <30mL/min
- Severe liver impairment
- Pregnant
- Documented history of heparin Induced Thrombocytopenia
- The simplified Pulmonary Embolism Severity Index (PESI) Score: The patient must not have any of the following criteria.
- Age >80
- History of cancer
- History of chronic cardiopulmonary disease
- Heart rate greater than or equal to 110
- Systolic BP < 100 mm Hg
- Oxygen saturations < 90%
How long should anticoagulation continue?
Up to 3 months: In patients with reversible causes, such as immobilisation or trauma.
Up to 6 months: In patients with right ventricular dysfunction or continued symptom.
Long Term: In patients with an unprovoked PE or risk factors (eg., malignancy or antiphospoholipid syndrome).
References
- Kahn SR., de Wit K. Pulmonary Embolism. NEJM 2022;387:45-57
- Tarbox AK., Swaroop M. Pulmonary Embolism. Int J Crit Illn Inj Sci. 2013 Jan-Mar; 3(1): 69–72
- Kline JA et al. Prospective multicenter evaluation of pulmonary embolism rule-out criteria. J Thromb Haemost 2008;6:772-780
- Ackerly I et al. Which age adjusted D-dimer cut-off performs best? EMA Vol 29, Issue 5, October 2017
- Flores J et al. Clinical usefulness and safety of an age-adjusted D-Dimer cut-off levels to exclude pulmonary embolism: a retrospective analysis. Intern Emerg Med 2016;11:69-75
- Verma N et al. Age-adjusted D-dimer cut-offs to diagnose thromboembolic events: validation in an emergency department. Med Klin Intensivmed Norfmed. 2014;109:121-128
- Gupta A et al. Assessing a 2 D-dimer age-adjustment strategies to optimise computed tomographic use in ED evaluation of pulmonary embolism. Am J. Emerg. Med. 2014;32:1499-1502.
- Van der Hulle T et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017; 390:289-297.
- Stevens SM, et al. Antithrombotic therapy for VTE dis- ease: second update of the CHEST guide- line and expert panel report. Chest 2021; 160(6):e545-e608.
- Lyman GH, et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treat- ment in patients with cancer. Blood Adv 2021;5:927-74.
- Konstantinides SV, et al. 2019 ESC guidelines for the diag- nosis and management of acute pulmo- nary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 2020;41:543-603.
- van Es N et al. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 2014;124:1969-1975